All things in our environment including the cells in our bodies go through a natural process called oxidation. Take an apple turning brown, or a nail rusting for instance. These are results of oxidation taking place and studies have also co-related oxidation to our aging process.
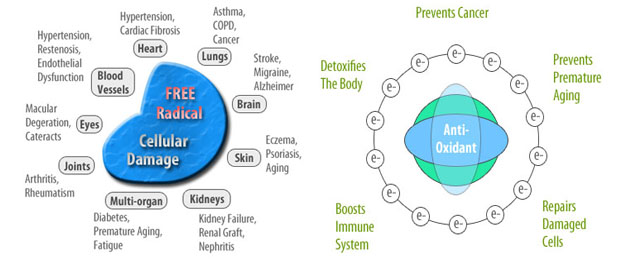
Antioxidants slow down the process of oxidation in our bodies. Free Radicals on the other hand don’t just promote the natural process of oxidation taking place in our bodies, they often inflict damage on our cell DNA, creating many health complications and increasing the risk of cancer.
Free Radical Damage
 Our bodies are made up of trillions of cells. Everyday, these cells are exposed to hordes of free radicals in our polluted environment. These free radicals steal electrons from our cells, damaging the DNA of the cells.
Our bodies are made up of trillions of cells. Everyday, these cells are exposed to hordes of free radicals in our polluted environment. These free radicals steal electrons from our cells, damaging the DNA of the cells.
Coupled with an acidic body and harmful carcinogens from cigarette smoke, you’ve got a prescription for cancer.
Role of Antioxidants
Antioxidants on the other hand, are free radical scavengers. They stabilize free radicals by donating electrons to them and also repair our damaged cells. Antioxidants are also known to prevent cancer by neutralizing cancer-causing free radicals before they do any damage to our cells.
Antioxidants are found in fruits that contain high doses of Vitamin C, vegetables such as brocoli and garlic, and green tea.

Oxidation Reduction Potential (ORP)
To measure how oxidative or antioxidant a substance or compound is, an Oxidation Reduction Potential(ORP) reader is used.
To make things simple to understand, solutions with a higher (more positive) reading are the “bad guys” while solutions with a higher (more negative reading) are the “good guys” and a good way to differentiate between antioxidants and free radicals is to picture Antioxidants as electron givers and Free Radicals as electron takers.
The more positive (a higher reduction potential) a solution, the more likely it is to “take electrons” from other agents(reducing other agents) it comes in contact with. The more negative (a lower reduction potential) a solution, the higher the tendency to “give electrons” to other agents.
Kangen Water is an abundant antioxidant source and produces a high negative ORP reading.